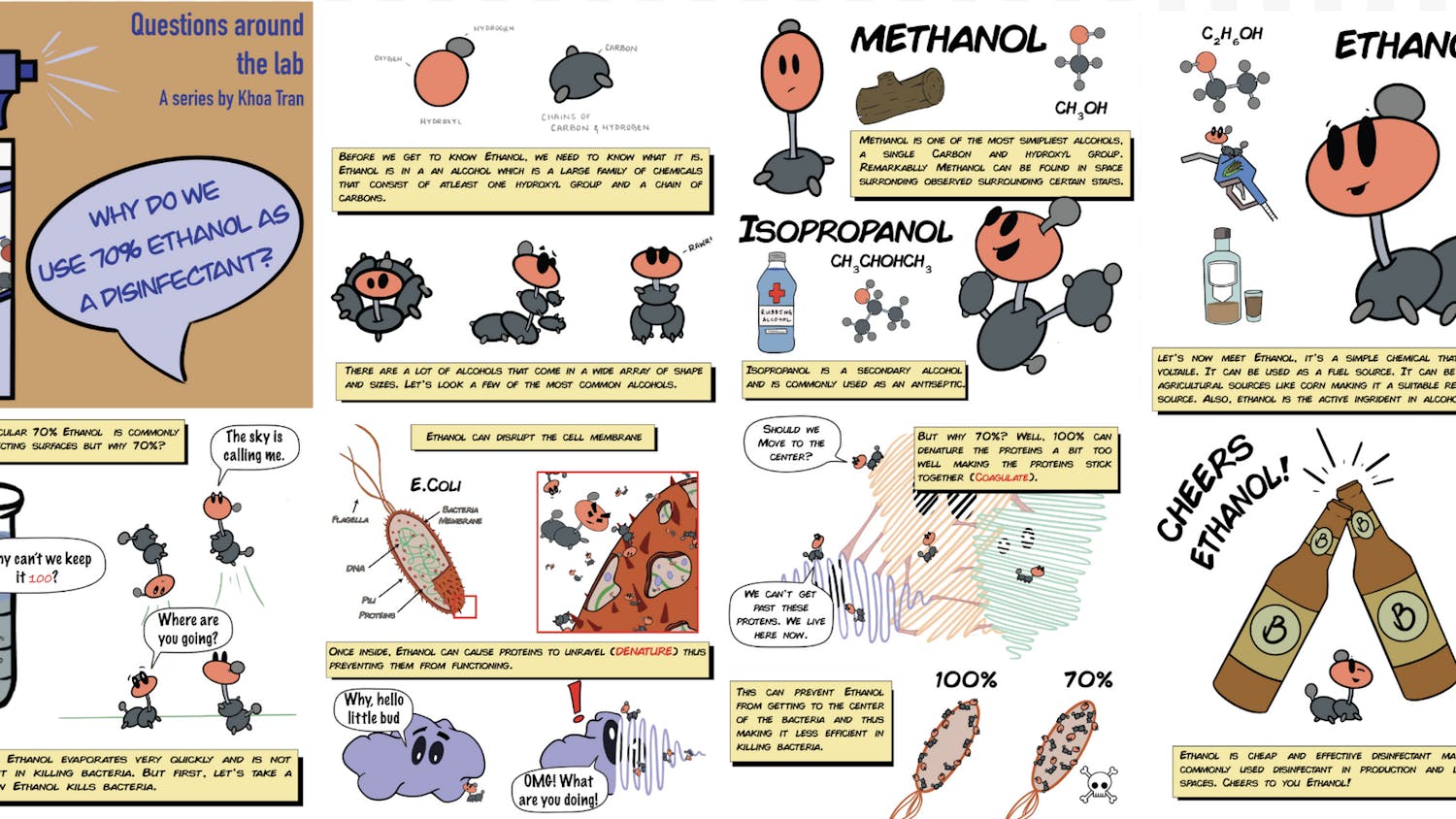
Responsible for nearly 10 million deaths worldwide in 2020, cancer consistently ranks among the leading causes of death each year. Brought on by genetic mutations and external risk factors, cancer is characterized by the transformation of normal cells into tumor cells. These mutated genes often encode for proteins responsible for regulating cell growth and cell death. When these processes are dysregulated, normal cells begin to proliferate uncontrollably and become cancerous.
The most commonly mutated gene in the RAS family, the Kirsten rat sarcoma viral oncogene, is one such gene that is commonly mutated in many cancers such as pancreatic ductal adenocarcinoma and colorectal cancer. This gene encodes the KRAS protein, which is activated when bound to guanosine 5’-triphosphate — a high-energy molecule commonly used in signaling pathways like these — and inactivated when bound to guanosine diphosphate, GTP’s lower-energy analog. When activated, KRAS binds to other molecules, transmitting signals that stimulate key cellular pathways, including those that promote cell growth.
One of these pathways, the RAS–RAF–MEK–ERK signalling pathway, regulates cellular processes involving proliferation, differentiation and survival. When mutations occur in the KRAS gene, levels of activated KRAS increase, forming nanoclusters in the plasma membrane — the barrier between the cell’s interior and exterior. These clusters promote downstream molecular interactions, amplifying the expression of relevant genes that drive cell proliferation and cancer progression. Because of their central role in these processes, RAS-family proteins like KRAS are prime targets in the development of cancer therapeutics.
DIRAS3, a RAS inhibitor naturally produced in the body, can directly bind to RAS proteins and impede the nanoclustering, ultimately inhibiting cancer cell growth in cancer lines caused by KRAS mutations. However, producing therapeutic molecules that can similarly effectively hinder cancer cell growth across mutant KRAS-driven cancers has proven challenging. Chemists at the University of Texas set out to analyze how DIRAS3 endogenously inhibits KRAS to inform the production of an exogenous molecule — one that could be administered to cancer patients to inhibit tumor growth. Their study found that certain engineered peptides derived from DIRAS3 can effectively disrupt KRAS nanoclusters in both cell culture and xenograft models.
To determine which molecular aspect of DIRAS3 disrupts KRAS clustering, the researchers generated four peptide models with slight variations: Compounds 1, 2, 3 and 4. Compounds 2 and 3 were notably cyclized, meaning their linear peptide chains were chemically linked to form ring-like structures.
The team used nuclear magnetic spectroscopy — a technique that reveals molecular behavior based on magnetic properties — to analyze how Compounds 3 and 4 bind to KRAS. Findings show that cyclization likely enhances DIRA3’s ability to bind the KRAS protein, potentially because cyclization reduces molecular disorder and stabilizes the peptide structure.
These findings were applied to KRAS nanoclusters. To do so, they used a chloroalkane penetration assay, a technique developed by members of the Kritzer lab in Tufts’ very own Department of Chemistry. This assay quantitatively measures how well an external agent penetrates cells. Using it, the team examined another modified DIRAS3 peptide: Compound 2, a similarly cyclized variant that can be synthesized in larger quantities.
The results confirmed that the cyclic DIRAS3 peptide successfully inhibited KRAS nanoclustering in cancer cells without reducing cell penetration, underscoring its ability to actually enter a cell to disrupt the clusters. Additional experiments demonstrated that treatment with Compound 2 significantly inhibited cancer cell growth and reduced mean tumor volume in both pancreatic and ovarian cancer cell and xenografts models.
These findings suggest that a cyclic DIRAS3-derived peptide, such as Compound 2, could serve as a promising therapeutic candidate for KRAS-driven cancers. While a comprehensive evaluation is needed to confirm its potential as a future target for cancer, this study highlights a potential for an alternative strategy to directly inhibiting KRAS signalling in KRAS-driven cancers.